Solid Acts Like a Liquid
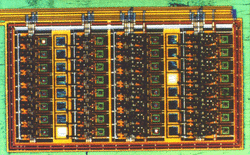
Photons and electrons. Optoelectronic chips of the future may use transition metal silicides.
Molecular beam epitaxy allows researchers to create thin slabs of materials with extremely precise control for use in the electronics industry and in basic physics research. A “substrate” crystal such as silicon serves as the base upon which atoms from a beam are deposited in layers. The deposited atoms tend to line up with those in the substrate, or at least in patterns strongly influenced by substrate atoms. Researchers have discovered many atomic configurations (“pseudomorphic phases”) that exist only in epitaxial films and not in the independently crystallized, or “bulk” material.
Physical properties can differ dramatically between bulk and epitaxial phases. For example, some of the transition metal silicides are bulk semiconductors but become metals under appropriate thin film conditions. Researchers hope to exploit that property to make microscopic metallic connections to semiconductor chips. Elio Moroni, of the Vienna Technical University, says that physicists must first understand and learn to control the many complicated atomic configurations available in these materials before they can be exploited.
Using computer simulations based only on basic quantum mechanics, Moroni and his colleagues studied elastic properties, which are closely related to electronic properties–the ones physicists ultimately need to control for industrial applications. They studied the effects of straining epitaxial crystal structures by varying the atomic spacing of the substrate. The team was surprised to find that variations over a range of 0.3 Å along certain directions with selected configurations of CoSi����, NiSi����, and FeSi2����2 cost no energy. The energy in the metal-silicon bonds and the total volume remained constant. Under these specific strains the materials seemed to react the way a liquid would–if squeezed in one direction, they would simply expand in another. The usual “harmonic” approximation for solids, by contrast, is that bonds act like coiled springs–as they are compressed or stretched, the energy increases with the square of the change in length. The authors explain the “supersoft” quality as a result of the many epitaxial phases available to these materials. As the bond lengths are forced to change, the crystals find alternate configurations with approximately the same energy and volume.
Hans von Känel, of the Swiss Federal Institute of Technology (ETH) in Zurich, calls the results “absolutely amazing,” since the elasticity ofepitaxial phases is usually completely normal, responding like a spring. He says the community had assumed that the elasticity of these heavily studied materials was well understood and expects the paper to stimulate many of his colleagues to begin trying to verify the Viennese group’s predictions.
#SolidFlow#LiquidLikeSolid#FlowingSolids#SolidBehavior#SolidLiquidity
#Pseudoliquid#SolidViscosity#FluidizedSolids#GranularFlow#SoftSolids
#SolidStateFlow#ViscoplasticSolids#DeformableSolids#SolidRheology
#SolidToLiquid#ThixotropicSolids#JammingTransition#YieldingSolids
#CreepingSolids#FlowableSolids


Comments
Post a Comment